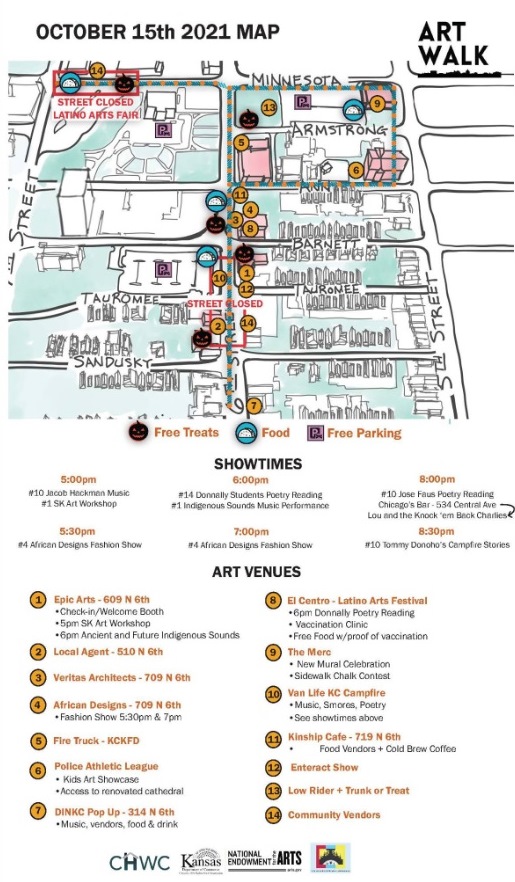
The Downtown Kansas City, Kansas, Art Walk will be from 5 p.m. to 9 p.m. Friday, Oct. 15, in the downtown Kansas City, Kansas, area.
The event will feature food, low riders, art and music. The general area is 6th and Sandusky to Minnesota Avenue. A low rider event will be near 6th and Minnesota.
Also at the event, participating sites will have a trunk and treat for kids.
A Latino Arts Festival will be held from 5 p.m. to 8 p.m. Friday at El Centro, 650 Minnesota Ave. The event at El Centro will include food, free to those who show proof of vaccination. There will be free COVID-19 vaccines available at the event at El Centro. A poetry reading from Donnelly College will take place at 6 p.m. at El Centro.
The Merc at 5th and Minnesota will have a new mural celebration.
Epic Arts at 609 N. 6th will have an art workshop, as well as music.
City Hall lobby, 701 N. 7th St., will be the site of the opening of an exhibit on the riverfront. The exhibit is by the Kansas City Design Center, in partnership with the Unified Government Planning and Urban Design Department. It depicts idea for riverfront development here. The exhibit will open at 4 p.m. Friday and will be open through 7 p.m. Friday during the Art Walk. Afterward, it will be open during regular City Hall hours.
For more information, visit the Downtown Shareholders site at https://www.facebook.com/DowntownShareholders/photos/a.549155951766867/5078693788813038.